Methanol has a high energy density. One litre of reformed methanol (CH3OH) contains 2.1 times more hydrogen than a litre of liquefied hydrogen (LH2).
Hydrogen bound in methanol thereby comes out very favourably in terms of energy content, volume and weight when compared with pure hydrogen as a liquid or compressed in gaseous form, or with battery operation.

Diesel
Diesel or marine gas oil, is practical in volume terms and very energy intensive. Environmentally, however, it is an undesirable fuel option which must be replaced with green alternatives as soon as possible.

Methanol
Running on methanol is twice as efficient as liquefied hydrogen. Tanks to contain it can be integrated in and follow the shape of a ship’s hull, thereby ensuring the desired cargo capacity.

Ammonia
Ammonia also has a high energy density, similar to methanol, but requires a rigid and more complex tank system owing to a higher risk potential.

Liquefied hydrogen
Liquefied hydrogen (LH2) requires a more complex tank system with a larger volume which weighs a lot because of the insulation needed to keep the temperature as low as -253°C.
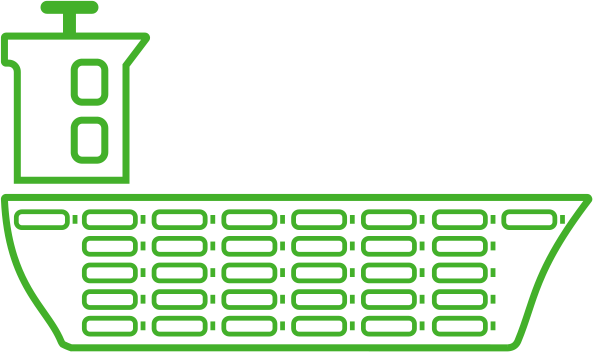
Compressed hydrogen
Hydrogen under pressure requires a large number of cylindrical tanks in steel or composite materials, which becomes a heavy system and takes a lot of space in the ship.
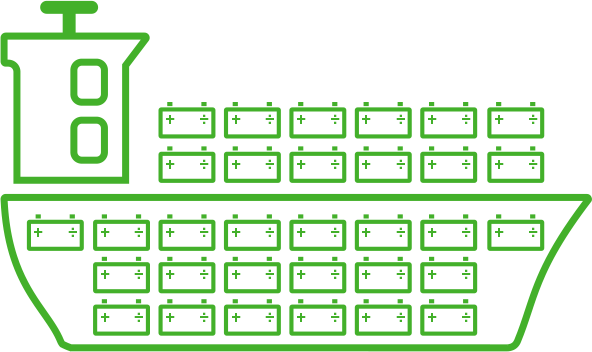
Battery operation
A cargo ship would have to fill virtually its entire hull with batteries to obtain sufficient energy for propulsion. Pure battery operation will only be appropriate over short distances.